Describe Two Examples of Compounds and Compare Their Properties
Environmental protection agency cincinnati ohio 45268 office of solid waste and emergency response us. They also support other cannabis compounds in producing desired effects.
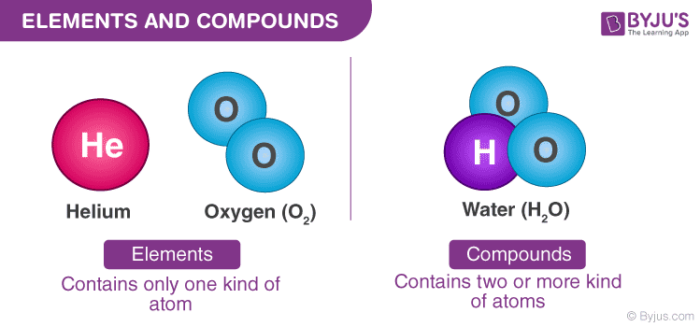
Definition Of Compounds Elements Examples Types Classification With Videos
Figure 230 A has additional examples of single bonds.

. Whether consuming cannabis for personal or medical use consumers are. Mechanical property maps more commonly known as Ashby maps have become a convenient manner of concentrating a large amount of information into one simple diagramThe first such map was proposed for metals by Weertman and therefore the name WeertmanAshby is sometimes usedThey constitute a valuable design tool and have been. Properties of Ionic and Covalent Compounds Ionic and covalent compounds differ in their properties because the particles in each of these two compounds are held together by different types of chemical bonds.
----- soil properties classification and hydraulic conductivity testing draft technical resource document for public comment sw-925 municipal environmental research laboratory office of research and development us. Homeschool parents will find that the engaging videos bring learning to life and make even the. In abstract algebra group theory studies the algebraic structures known as groupsThe concept of a group is central to abstract algebra.
Definition of Heterogeneous Mixtures. Aromatic compounds contain a cyclic hydrocarbon benzene C 6 H 6 with alternating double-bonds. A molecule can have multiple single bonds.
In some mixtures the initial. For example water H 2 O has two single bonds one between each hydrogen atom and the oxygen atom Fig. The characteristic properties of the whole RA B C where R marks their structural arrangement cannot even in theory be deduced from the most complete knowledge of the properties of A B and C in isolation or in other wholes which are not of the form RA B C.
Covalent compounds Ionic compounds composed of simple molecules. Compare the glucose and fructose molecules in the figure below. Can you identify their differences.
The suffix yne is generally used to describe. Examples for the nomenclature of alkenes include the name ethene used to describe the compound given by C 2 H 4 and Propene used to describe the compound given by C 3 H 6. The suffix ene is used to describe alkenes via IUPAC norms.
A mixture is a combination of two or more pure substances in which the original substances retain their chemical properties. These differences affect the properties of the two monosaccharides. Due to resonance structures the aromatic ring is extremely stable and does not undergo the typical reactions expected of.
Other well-known algebraic structures such as rings fields and vector spaces can all be seen as groups endowed with additional operations and axiomsGroups recur throughout mathematics and the methods of group theory have. In contrast to intramolecular forces such as the covalent bonds that hold atoms together in molecules and polyatomic ions intermolecular forces hold molecules together in a liquid or solidIntermolecular forces are generally much weaker than covalent bonds. The General formula of alkynes is C n H 2n-2.
The only differences are the positions of some of the atoms. Terpenes do more than provide flavor and aroma. Environmental protection agency washington dc.
This is called the entourage or ensemble effect and its the reason these aromatic compounds have become such a critical area of cannabis research. They are named much like alkenes but with the ending yne. Course Summary This online homeschool course is all you need to teach 6th grade physical science.
Carbohydrates have many isomers because of the arrangement of the ce-OH groups in their structures. Table compares and contrasts the properties of ionic and covalent compounds. Sometimes two covalent bonds are formed between two atoms by each atom sharing two electrons for a total of four shared.
The properties of alkynes are quite similar to those of alkenes. The properties of liquids are intermediate between those of gases and solids but are more similar to solids.

Elements Compounds And Mixtures Graphic Organizer Graphic Organizers Learning Stations Heterogeneous Mixture

Matter Atoms Elements Molecules And Compounds Anchor Posters Atoms And Molecules For Kids Science Poster Science Display

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Study Chemistry

Pure Substances And Mixtures Venn Diagram Examples Venn Diagram Worksheet Venn Diagram Template

Elements Compounds And Mixtures Odd One Out Worksheet Physical Science Lessons Middle School Science Compounds And Mixtures

Objectives I Can Compare The Properties Of Ionic And Covalent Compounds With A Venn Diagram I Can Practice Dra Chemical Bond Chemical Changes Covalent Bonding

Gcse Chemistry Atoms Elements And Compounds Mixtures Complete Revision Summary And Notes Gcse Chemistry Compounds And Mixtures Gcse Chemistry Revision

Compound Vs Mixture Difference And Comparison Diffen Compounds Science Teaching Chemistry Chemistry Classroom

Organic And Inorganic Compounds Venn Diagram Venn Diagram Student Writing Inorganic Compound

Difference Between Electrophile And Nucleophile Comparison Summary Teaching Chemistry Chemistry Education Chemistry Study Guide

Differences Between Elements Compounds Mixtures Compounds And Mixtures Elements Compounds And Mixtures Compounds Science

Compounds In Chemistry Overview Examples What Is A Compound Video Lesson Transcript Study Com

Difference Between Compound And Mixture Compounds And Mixtures Basic Concepts Chemistry

Differences Between Elements Compounds Mixtures Compounds And Mixtures Elements Compounds And Mixtures Compounds Science

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Teaching Chemistry

Elements Compounds And Mixtures Doodle Notes Science Doodle Notes Teaching Chemistry 6th Grade Science Chemistry Classroom

Classification Of Matter Concept Map Teaching Chemistry Chemistry Basics Chemistry Education

Comments
Post a Comment